Question: For a particular gaseous system it has been determined that energy is given by:

In this equation P is expressed in Pascals and V is in m3. The system is initially in the state A (P=0.2MPa, and V=10 L). The system is then transfered to the state B (P=0.2MPa, V=30 L) along the parabola
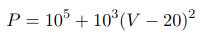
In this equation volume is in litres and pressure is in Pascals. Calculate Q and W for this process. Also find the equation of the adiabats in the P-V plane (i.e., find the form of the curves P=P(V) such that dQ=0 along the curves).
Order step by step solution of this question written in pdf format:
Solution ID: StTh-HW1-3
Solution of this question will be sent to your email account within 8 hours.
$29.99
Order step by step solution of this question written in pdf format:
Solution ID: StTh-HW1-2
Solution of this question will be sent to your email account within 8 hours.
$19.99
For any inquiry about this solution before and/or after purchase please fill in the following form and submit it to Detailed Solution.