Question:
Consider a simple model for a charge-transfer reaction that describes it as a motion along some reaction coordinate x, i.e., a Markus model. The energies of reactants and products are then given by
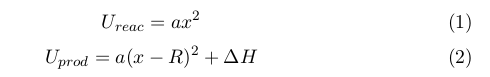
where ∆H is an enthalpy of the reaction.
a) Find the expression for the activation energy in terms of a, R, and ∆H.
b) What is the activation energy in the limits of R<< 1 and R>> 1?
c) At what enthalpy of the reaction the activation barrier for the reaction disappears?
d) When the heat of reaction is small the activation energy can be written as,

Express the parameters α and β in terms of a and R.
Solution:
a) Activation energy is the difference between the energy at intersection
point of reactants and products curves…….
Order step by step solution of this question written in pdf format:
Solution ID: Reaction-Dynamics-Fi2-2
Solution of this question will be sent to your email account within 8 hours.
$54.99
For any inquiry about this solution before and/or after purchase please fill in the following form and submit it to Detailed Solution.